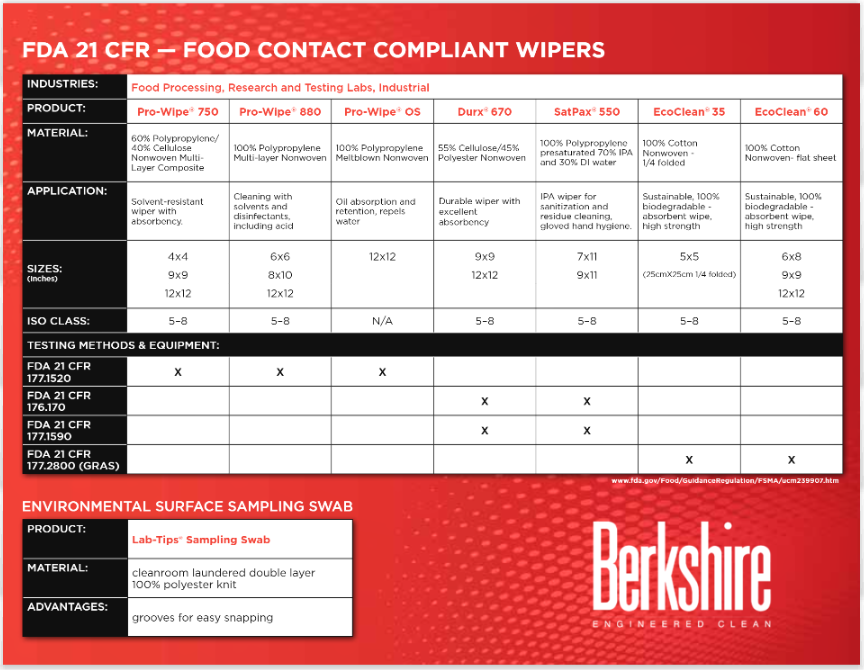
In our industry, a couple of acronyms are important to understand: FDA, GRAS, and CFR. This article explains what these stand for and their implications for products manufactured for inclusion in and contact with food items and packaging.
The federal Food and Drug Administration (FDA) was established following the passage of the 1906 Pure Food and Drugs Act – later superseded by the Federal Food, Drug, and Cosmetic Act (‘the Act’) – and is now the oldest consumer protection service in the US. Its core mission is to enforce laws relating to public health and, in this role, the agency regulates food ingredients and additives, chemicals, pharmaceuticals, biological products, medical devices, radiation-emitting devices, toxicological research, and veterinary pharmaceuticals and devices. Furthermore, it is responsible for reviewing product labeling, creating and disseminating industry-focused educational and advisory materials, and instituting certification programs. In tandem, the Office of Regulatory Affairs (ORA) works with the FDA to carry out investigations and laboratory analysis, and gathers data to support regulatory enforcement via injunctions, warning letters, product recalls, and other secondary/tertiary procedures.
Although created with a domestic focus, the agency has developed partnerships with foreign counterparts in response to the globalization of food chains through the growth of multinational corporations with worldwide markets. Today, the agency bases its work on the following premise: ‘Good public health protection demands more than a solid inspection program to manage imports. Faced with this unprecedented increase in products from abroad, the FDA has relied on two primary strategies for helping to assure the quality of these products: harmonization of standards through multilateral fora, and two-party (bilateral) agreements with other nations. In general, the FDA and other nations have entered into harmonization initiatives to assure product quality while at the same time conserving scarce human and financial resources and improving the efficiency of their operations. When nations can agree on scientific standards for establishing the safety, effectiveness, and manufacturing quality of pharmaceutical products, or on standards that help ensure that high-quality medical devices are produced, everyone wins.’(1)
One of the central tenets of safety for food contact surface products is the concept of ‘GRAS.’
According to the FDA, ‘GRAS’ is an acronym for the phrase ‘Generally Recognized As Safe. Under sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act (the Act), any substance that is intentionally added to food is a food additive, that is subject to premarket review and approval by FDA, unless the substance is generally recognized, among qualified experts, as having been adequately shown to be safe under the conditions of its intended use, or unless the use of the substance is otherwise excepted from the definition of a food additive.’(2)
The FDA notes that ‘general recognition of safety through experience based on common use in foods requires a substantial history of consumption for food use by a significant number of consumers.’(3) For materials used in contact with foods this means there are two routes to obtaining GRAS certification: testing and common use history. In laboratory testing, materials are certified ‘through scientific procedures […] based upon the application of generally available and accepted scientific data, information, or methods […] as well as the application of scientific principles, and may be corroborated by the application of unpublished scientific data, information, or methods.’(4) Alternatively, if a chemical or food ingredient has been used commonly in manufacture since 1958 or earlier it can be certified GRAS ‘through experience based on common use in food Under 21 CFR 170.30(b).’(5)
To understand what these titles and sections refer to, it’s useful to examine what is meant by the CFR and how it relates to food contact surfaces.
The codes that indicate GRAS status pertain to regulatory guidelines compiled in the Code of Federal Regulations (CFR). The CFR documents all regulations issued from executive branches and agencies of the federal government. Published to the Federal Register, it is available in printed form in a set of 200 volumes or it is also accessible online here. With 50 areas of subject matter (‘titles’), navigating this codification is complex, although only a few of these titles relate directly to food contact surfaces. In each case, the content is sub-divided into title (a broad subject area), chapter (the rules of individual agency), part (the rules on a single program or function), section (one provision of program/function rules), paragraphs and sub-paragraphs (detailed, specific requirement). Here is an example for regulations pertaining to ‘Unavoidable Contaminants in Food For Human Consumption and Food-Packaging Material’: Title 21 (Food and Drugs), Chapter 1 (Food and Drug Administration, Department of Health and Human Services), Subchapter B (Food for Human Consumption), Part 109 (Unavoidable Contaminants in Food for Human Consumption and Food-Packaging Material). For additional clarity in the format of CFR citations, the Federal Register archives specify an example as follows: ‘Cite the FR by: volume, page no. and date: 64 FR 34567 (June 5, 1999)’(6)
Our industry is regulated by myriad rules and guidelines, and a thorough understanding of both what they are and how they impact our products and processes is critical to optimal performance. We hope that this article is useful in comprehending the role of the FDA, the use of GRAS certification, and the operation of the CFR in relation to food contact surfaces. Please let us know if this information is helpful, and we welcome questions in the comments.
References:
- https://www.fda.gov/about-fda/changes-science-law-and-regulatory-authorities/fda-goes-global
- https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras
- ibid
- ibid
- ibid
- https://www.archives.gov/federal-register/tutorial/online-html.html#introduction